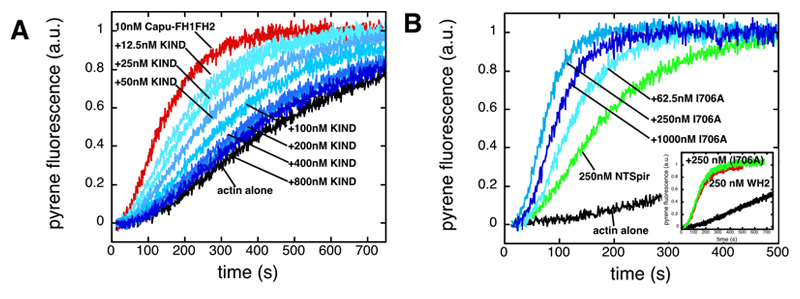
Interaction between Spir and Capu affects actin nucleation. (A) The Spir-KIND domain inhibits actin nucleation by Capu-family formins. We induced polymerization by mixing pyrene-labeled actin with Mg2+, K+ and Capu-FH1FH2. Addition of KIND to Capu-FH1FH2 before mixing with actin potently inhibited nucleation activity in a dose dependent manner. Protein concentrations: Actin – 4 mM (5% pyrene labeled), Capu-FH1FH2 – 10 nM, KIND – as indicated. (B) Capu enhances actin nucleation by Spir. We mixed several concentrations of a nucleation incompetent mutant of Capu-FH1FH2 (Capu-FH1FH2(I706A)) with the N-terminal half of Spir (NTSpir), which contains the KIND domain and four WH2 domains (only three concentrations are shown for clarity). Nucleation activity of NTSpir was increased by Capu-FH1FH2(I706A) until the proteins were approximately equimolar and then activity decreased. Inset. Capu-FH1FH2(I706A) does not enhance the activity of the WH2 cluster alone, indicating that an interaction between the KIND domain and the formin is necessary. Protein concentrations: Actin – 4 mM (5% pyrene labeled), NTSpir and WH2 – 250 nM, Capu-FH1FH2(I706A) –as indicated. |